AstraZeneca jab guidance change for under-30s has not caused hesitancy – study
The Medicines and Healthcare products Regulatory Agency has said there is a possible link between the vaccine and ‘extremely rare’ blood clots.

The decision to offer the under-30s an alternative to the AstraZeneca vaccine over blood clotting concerns has had no impact on people’s intention of getting the jab, new research suggests.
New UK guidance was issued on April 7 recommending that people aged 18 to 29 should be offered the Pfizer or Moderna vaccines after the Medicines and Healthcare products Regulatory Agency (MHRA) said there is a possible link between the AstraZeneca jab and “extremely rare” blood clots.
Several European countries including France, Germany and Italy suspended use of the vaccine in March over the link, although they later said they would resume its rollout.
University of Stirling researchers have been collecting data for a wider project on fear and concerns related to Covid-19 and they examined whether public concern about the AstraZeneca jab led to “vaccine hesitancy”.
They carried out a survey after news of the European suspensions emerged in mid-March, and they found no drop in the proportion of people who said they intended to get the vaccine.
Researchers carried out another survey on April 9 after guidance on vaccinating the under-30s changed and found only a slight change in people’s intentions.
On April 9 they found that 85.7% of respondents said they intended to get the vaccine compared to 86.1% on March 17.
They also found little change in the 30 to 40 age group, who will continue to be offered the AstraZeneca vaccine.
Dr David Comerford, of Stirling’s Behavioural Science Centre, said: “I was surprised – I thought we would see a change in response following the UK regulator’s new guidance.
“Perhaps not from the under-30s, who would be offered different vaccines, but if you were 31–35, say, we would have expected hesitancy.
“This is not to say that people were not concerned by the news. Google Trends data shows increasing search activity for the terms ‘vaccine’ and ‘safe’ coinciding with the headlines.
“Also, on April 9 – the day of our data collection – the president of the Royal College of Emergency Medicine, Dr Katherine Henderson, reported that all A&E departments witnessed an increase in the number of people reporting concerns after having the AstraZeneca vaccine.
“These concerns did not translate into mistrust of the vaccination programme in the UK, however.”
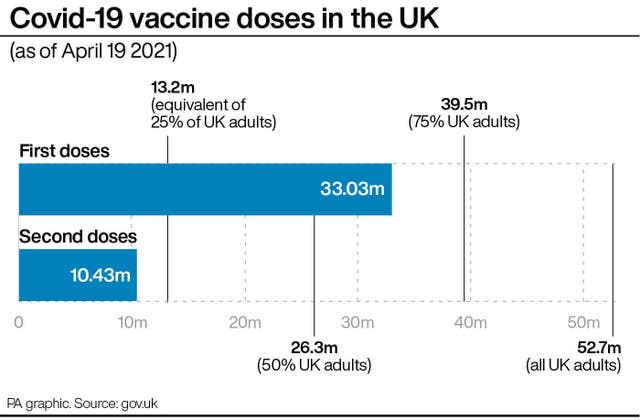
Researchers found that for those in the 30-39 age group, 85.3% said they intended to take the vaccine after the change in guidance, compared to 87.3% before, while 9.8% said they would refuse it compared to 9.9% before the guidance changed.
Data was collected from 502 people around the UK on April 9 and the research is published on the Open Science Framework (OSF) website.
It has not yet been peer reviewed but will be in due course.
The MHRA has said the benefits of the AstraZeneca vaccine still outweigh the risks overall.
It said the balance of risk for the jab is very favourable for older people but “more finely balanced” for younger age groups, who do not tend to suffer serious Covid illness.
Up to March 31, the MHRA in the UK received 79 reports of blood clots accompanied by low blood platelet count, all in people who had their first dose of the vaccine, out of around 20 million doses given.
Of these 79 people, 19 had died, although it has not been established what the cause was in every case.
Of the 19 who died, three were under the age of 30, the MHRA said.





