All under-40s to be offered alternative to AstraZeneca vaccine
Up to April 28, the MHRA in the UK had received 242 reports of blood clots accompanied by low blood platelet count, all in people who had AstraZeneca.

All under-40s are to be offered an alternative to the Oxford/AstraZeneca Covid vaccine in a precautionary move.
The Joint Committee on Vaccination and Immunisation (JCVI) and the Medicines and Healthcare products Regulatory Agency (MHRA) are expected to say there are no new safety concerns after previous data showed the AstraZeneca jab was linked to very rare blood clots.
Previously, the MHRA has said the balance of risk for the AstraZeneca vaccine against Covid is very favourable for older people but “more finely balanced” for younger groups, who do not tend to suffer serious coronavirus illness.
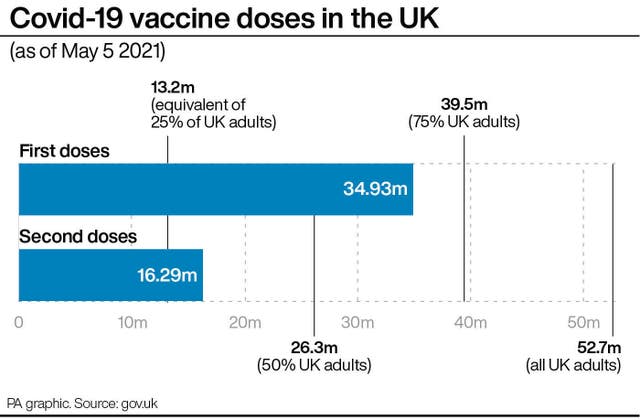
Up to April 28, the MHRA had received 242 reports of blood clots accompanied by low blood platelet count in the UK, all in people who had AstraZeneca, out of around 28.5 million doses given.
These clots occurred in 141 women and 100 men aged from 18 to 93, and the overall case death rate was 20%, with 49 deaths. Six cases have been reported after a second dose of the vaccine.
A particular type of brain blood clot – cerebral venous sinus thrombosis (CVST) – was reported in 93 cases (with an average age of 47), and 149 had other major thromboembolic events (average age 55) accompanied by low blood platelet count.
The MHRA and JCVI have both said that the benefits of the AstraZeneca vaccine continue to “outweigh the risks for the vast majority of adults”.





