Thailand’s PM receives AstraZeneca jab as Asia mostly backs treatment
Concerns have been raised in Europe about blood clots with some countries suspending its use.
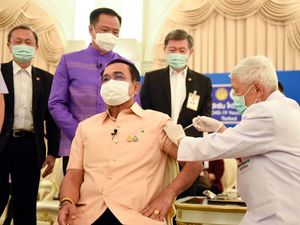
Thailand’s prime minister received a jab of the Covid-19 vaccine manufactured by AstraZeneca, as much of Asia shrugged off concerns about reports of blood clots in some recipients in Europe, saying that so far there is no evidence to link the two.
Many countries using the vaccine also said the benefits from inoculation far outweighed possible risks, even as parts of Europe suspended it pending investigation of potential side effects.
AstraZeneca has developed a manufacturing base in Asia, and the Serum Institute of India, the world’s largest vaccine maker, has been contracted by the company to produce a billion doses of the vaccine for developing nations.
Hundreds of millions more are to be manufactured this year in Australia, Japan, Thailand and South Korea.
“There are people who have concerns,” Thai prime minister Prayuth Chan-ocha said after he received the first dose of the AstraZeneca vaccine.
“But we must believe doctors, believe in our medical professionals.”

Thailand last week was the first country outside Europe to temporarily suspend using the AstraZeneca vaccine.
Indonesia followed on Monday, saying it was waiting for a full report from the World Health Organisation regarding possible side effects.
But Thailand’s health authorities decided to go ahead with AstraZeneca, with Mr Prayuth and members of his Cabinet receiving the first jabs.
A large number of European countries, including Germany, France, Italy and Spain, suspended use of the AstraZeneca vaccine Monday over reports of dangerous blood clots in some recipients, though the company and international regulators say there is no evidence the shot is to blame.
The EU’s drug regulatory agency called a meeting for Thursday to review experts’ findings on the AstraZeneca shot and to decide whether action needs to be taken.
Other countries in the Asia-Pacific region also said they would press ahead with vaccination programmes.
In the Philippines, presidential spokesperson Harry Roque said his country would not suspend usage because the benefits outweighed any risks.
The country has so far received 525,00 doses of the AstraZeneca vaccine under the World Health Organisation’s Covax arrangement and has administered 12,788 doses so far.

Several million more doses have been ordered by the government and private companies.
“There is still no clear data that shows that the blood clotting was caused by AstraZeneca.
“If such data will come out, maybe we will also stop the use of AstraZeneca,” Mr Roque said.
“As of now, our experts are saying again that the benefits we get from using AstraZeneca are larger than the side effects of this vaccine.”
Australian health minister Greg Hunt said his country would not suspend vaccinations.
Australia has vaccinated about 200,000 people so far and plans to import and manufacture 70 million vaccine doses from AstraZeneca.
“The government clearly, unequivocally, absolutely supports the AstraZeneca rollout, clearly, unequivocally, absolutely.
“And the reason why is very simple — it will help save lives and protect lives, and it’s done so on the basis of the medical advice,” Mr Hunt told Parliament.

Australia’s chief medical officer, Paul Kelly, said there was no evidence so far that the vaccine causes blood clots.
“Blood clots happen, they happen in Australia fairly commonly,” he said.
“But, from my perspective, I do not see that there is any specific link between the AstraZeneca vaccine and blood clots, and I’m not alone in that opinion.”
By far the largest user of the AstraZeneca vaccine is India.
India is using two vaccines, the AstraZeneca shot made by Serum Institute of India, and another one by Indian vaccine maker Bharat Biotech, to immunise its vast population.
Of the more than 25.6 million people in India who have received at least one shot of a vaccine, over 23.4 million have received the AstraZeneca shot, according to government data.
Health officials told the Press Trust of India news agency on Saturday that a total of 234 adverse events, including 71 deaths, had been reported after receiving either vaccine, but that no causal link had been found.
The government is now reviewing the cases for a final assessment.

Serum Institute of India, the world’s largest vaccine maker, has been contracted by AstraZeneca to make a billion doses of vaccine for developing nations.
By March 4, India had exported over 48.1 million doses of vaccine, including 11.9 million doses to Covax and 28.8 million doses as commercial exports, according to government data.
Meanwhile, health activists and medical ethics experts in India have warned that India’s systems for monitoring any harmful side effects are too lax.
With the exception of a few countries, such as Singapore and India, Asian nations have been quite slow in getting their populations vaccinated.
Most of the nations, including Australia, New Zealand and Thailand, have been relatively successful in containing the spread of Covid-19.
Thailand has ordered just enough vaccine from AstraZeneca and China to cover about half its population this year and has so far managed to inoculate around 50,000 people in high-risk groups.
Meanwhile, China has approved a new vaccine for emergency use, developed by the head of its Centre for Disease Control, adding a fifth shot to its arsenal.
Gao Fu, the head of China’s CDC, led the development of a protein subunit vaccine that was approved by regulators last week for emergency use, the Chinese Academy of Sciences’ Institute of Microbiology said.
The vaccine was developed jointly by Anhui Zhifei Longcom Biopharmaceutical and the Chinese Academy of Sciences. The team finished phase 1 and 2 clinical trials in October and is conducting the last phase of trials in Uzbekistan, Pakistan and Indonesia, according to the statement.
The vaccine was approved for use in Uzbekistan on March 1. It is a three-dose shot that is spaced out with a month between shots, a company spokesperson said.





