Moderna to provide 500m vaccine doses for Covax programme
The advance purchase agreement comes just days after the World Health Organisation announced emergency approval for the vaccine.

Moderna and vaccine promoter Gavi have announced a deal by which the pharmaceutical company will provide up to 500 million doses for the UN-backed Covax programme by the end of 2022.
The advance purchase agreement comes just days after the World Health Organisation (WHO), after weeks of delays, announced emergency approval for the Moderna vaccine that will pave the way for its rollout in Covax.
The UN-backed programme aims to get coronavirus vaccines to needy people in low and middle-income countries.
However, deliveries are not set to begin until the fourth quarter of this year, and the vast majority of the doses in the deal – 466 million – are planned for next year, with the remaining 34 million expected this year.
Financial terms were not disclosed.
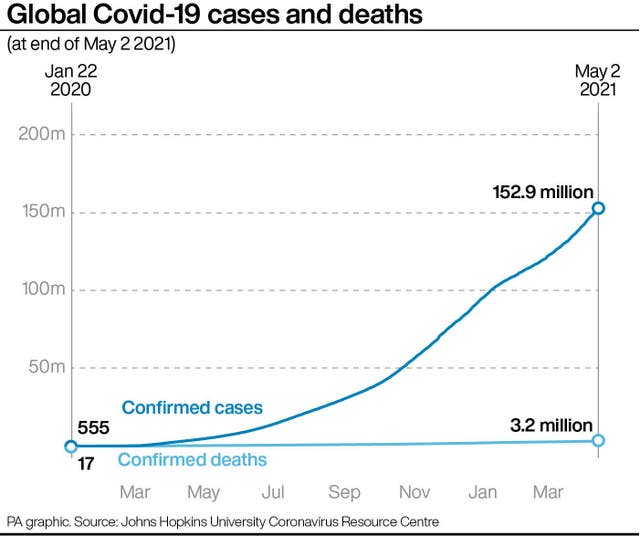
Many experts say the Covid-19 crisis is acute now, with India in particular facing an unprecedented surge in cases.
The Moderna vaccine has generally been considered among the most effective so far in combating new variants, such as the one that is spreading in India.
The arrangement means that Massachusetts-based Moderna can join the Covax rollout that already includes vaccines from Oxford/AstraZeneca, which has the biggest role so far in the programme, and Pfizer/BioNTech, which has committed far fewer doses to it.
Supplies of the AstraZeneca vaccine for Covax that are being produced in India have been limited in recent months as the New Delhi government and the Indian subcontractor divert much of that production to combating the devastating outbreak at home.
The Coalition for Epidemic Preparedness and Innovation, a public-private partnership that co-manages Covax with Gavi and the WHO, made an early investment into the Moderna vaccine as the pandemic arose – and the first official link-up between the company and the programme has come nearly 18 months into the pandemic.
The WHO go-ahead for an emergency use listing for Moderna’s vaccine, announced late on Friday, took many months because of delays that the WHO faced in getting data from the manufacturer.

Many countries without their own advanced medical regulatory and assessment offices rely on the WHO listing to decide whether to use vaccines.
UN children’s agency Unicef also uses the listing to deploy vaccines in an emergency like the pandemic.
Moderna has struck supply agreements with many rich countries, which will have already received millions of doses of the vaccine.
Gavi also announced that Sweden’s government has committed to donate one million doses of the Oxford/AstraZeneca vaccine “to help Covax urgently address immediate-term supply delays”.





